by codm | Oct 16, 2023 | Newsletter
Update on LEQEMBI Investigational Subcutaneous Formulation
For Print (PDF)
Eisai Co. Ltd (Headquarters: Tokyo, CEO: Haruo Naito, “Eisai”) announced today that the company will present new data from the phase 3 Clarity AD study for its Alzheimer’s disease (AD) treatment LEQEMBI® (lecanemab-irmb) 100 mg/mL injection for intravenous use and new data on the subcutaneous formulation in development at the 16th annual Clinical Trials on Alzheimer’s Disease (CTAD) conference. The conference will be held in Boston, Massachusetts, United States and virtually from October 24 to 27, 2023. In addition to the data presented on Eisai’s anti-amyloid beta (Aβ) protofibril* antibody LEQEMBI, phase 1 data for E2511, an investigational tropomyosin receptor Kinase A (TrkA) positive allosteric modulator (PAM), will be presented as well as other research from the company’s AD pipeline. At the conference, Eisai will present data and research in five oral and ten poster presentations. BioArctic will give an oral presentation on lecanemab.
Late-Breaking Symposium 4 – Lecanemab for early Alzheimer’s Disease: Long-Term Outcomes, Predictive Biomarkers, and Novel Subcutaneous Administration
- In a late-breaking symposium on October 25 from 17:25-18:05 EDT, Eisai will present the latest data from the Clarity AD optional tau PET longitudinal substudy. The presentation will include a post-hoc analysis of the low and intermediate + high-tau subgroups, with the low-tau subgroup representing early stages of disease studied specifically in the phase 3 core study, and the open-label extension study. An update on the investigational subcutaneous formulation, including interim safety and effect on amyloid in the brain measured by amyloid PET, will be provided.
- Distinguished faculty members Christopher van Dyck M.D., Keith Johnson M.D. and Reisa Sperling M.D. will discuss the findings in a panel led by Michael Irizarry, M.D., MPH, Eisai.
- A live webcast of this symposium can be viewed on the Eisai Co., Ltd. website.
“Alzheimer’s disease is a progressive and relentless condition that requires early diagnosis1,2,3 and continued treatment. LEQEMBI supports neuronal function in Alzheimer’s disease1,4,5 by clearing highly toxic protofibrils3,4 that can continue to cause neuronal injury and death well after plaques are cleared,”5,6,7,8 said Michael Irizarry, MD, MPH, Senior Vice President, Clinical Research, Neurology, Deputy Chief Clinical Officer, Clinical Evidence Generation, Eisai. “We look forward to sharing the new LEQEMBI low-tau subgroup data and subcutaneous data at CTAD 2023.”
Other major oral presentations include:
- Lecanemab: Binding profiles of lecanemab and donanemab to different amyloid-beta species (OC19, presentation by BioArctic).
- E2511, a novel TrkA modulator, engages its CNS cholinergic target in a phase 1 clinical study (OC34).
- Novel CSF tau biomarkers can be used for disease staging of sporadic Alzheimer’s disease (OC2).
The full list of presentations about Eisai assets and research follows.
■ Late Breaking Symposium 4
The Symposium title: Lecanemab for Early Alzheimer’s Disease: Long-Term Outcomes, Predictive Biomarkers and Novel Subcutaneous Administration from 17:25 to 18:05 EDT on October 25, 2023.
| Presentation Title |
| Clarity AD: Review of the Mechanism-Based Rationale and Results of the Lecanemab Phase 3 Trial |
| Biomarker Assessments from Clarity AD: A Focus on Downstream Implications of Targeting Protofibrils* and Tau as a Predictive Biomarker |
| Lecanemab for the Treatment of Early Alzheimer’s Disease: The Extension of Efficacy Results from Clarity AD |
| Preliminary Update on Lecanemab Safety in Clarity AD Open-Label Extension, Including Subcutaneous Formulation |
| Panel discussion, Q&A |
■ Oral Presentations
Asset/Project,
Presentation Time (EDT) |
Presentation Number, Title |
Lecanemab
October 26 (Thu) 14:50-15:05 |
OC19
Binding Profiles of Lecanemab and Donanemab to Different Amyloid-beta Species
(presentation by BioArctic) |
Lecanemab
October 26 (Thu) 17:05-17:45
(Late breaking symposium 6) |
Presentation 3 in Late breaking symposium 6
Aβ42/Aβ40 and Phospho-tau217 Concentration Ratios Increase the Accuracy of Amyloid PET Classification in Preclinical Alzheimer’s Disease |
E2511
October 27 (Fri) 14:45-14:55 |
OC34
E2511, a Novel TrkA Modulator, Engages its CNS Cholinergic Target in a Phase 1 Clinical Study |
Biomarker
October 25 (Wed) 8:45-9:00 |
LB7
PrecivityAD2™ Blood Test: An Analytically and Clinically Validated Test Combining p-Tau217/np-Tau217 and Aβ42/40 Ratios to Identify Brain Amyloid
(presentation by C2N) |
Biomarker
October 25 (Wed) 11:35-11:50 |
OC2
Novel CSF Tau Biomarkers Can Be Used for Disease Staging of Sporadic Alzheimer’s Disease |
AD general
October 27 (Fri) 11:45-12:00 |
OC29
AI-based Enrichment Tools to Increase Efficiency of Alzheimer’s Disease Clinical Trials |
| Asset/Project |
Presentation Number, Title |
| Lecanemab |
P018
Recruitment Source, Eligibility and Reason for Prescreen-Fail Across Sex, Race and Ethnicity: Preliminary Analysis of Prescreening Data from the AHEAD Study |
| Lecanemab |
P045
ARIA by Clinical Subgroup and Baseline Amyloid PET Centiloid Levels from the Lecanemab Clarity AD |
| Lecanemab |
LP011
Impact of a Site Supplemental Funding Program to Alleviate Recruitment Burden: Experiences in the Preclinical Alzheimer’s Disease AHEAD Study |
| E2511 |
P044Safety and Pharmacokinetics of Multiple Ascending Doses of E2511, a Novel TrkA Allosteric Modulator, in Healthy Volunteers |
| Biomarker |
P070
Systematic Literature Review of the Clinical and Non-clinical Value of Imaging and Fluid Biomarker Testing to Diagnose, Identify and Monitor Patients with Alzheimer’s Disease |
| AD general |
P032
Planning the Next Generation of Alzheimer’s Disease Clinical Trials Using Diverse Patient-level Database from the Critical Path for Alzheimer’s Disease (CPAD) Consortium |
| AD general |
P033
Critical Path for Alzheimer’s Disease (CPAD) Consortium: Data-Driven Solutions for Clinical Trial Design and Informed Decision Making |
| AD general |
LP003
Implications of Missing Data and Dropouts in Randomized Clinical Trials in Early Alzheimer’s Disease |
| AD general |
P145
Age-Specific Relative Comorbidity Burden of MCI: a US Database Study |
| AD general |
P179
Development of a Mild Cognitive Impairment Risk Prediction Model Using Electronic Health Record Data |
This release discusses investigational uses of agents in development and is not intended to convey conclusions about efficacy or safety. There is no guarantee that such investigational agents will successfully complete clinical development or gain health authority approval.
*Protofibrils
- One of the AD pathological features is the accumulation of clusters (plaques) of amyloid beta (Aβ) in the brain. The formation of these plaques is the result of a continuous process by which individual Aβ proteins join together, latching onto each other, one at a time, like adding links to a chain.9 In the early part of this process these small chains of Aβ are soluble and are toxic to the nerves within the brain.10,11
- The most toxic of the soluble chains is called a protofibril.3 Protofibrils are believed to contribute to the brain injury that occurs with AD and are considered to be the most toxic form of Aβ, having a primary role in the cognitive decline associated with this progressive, debilitating condition.1,2
- Protofibrils cause injury to neurons in the brain, which in turn, can negatively impact cognitive function via multiple mechanisms, not only increasing the development of insoluble Aβ plaques but also increasing direct damage to brain cell membranes and the connections that transmit signals between nerve cells or nerve cells and other cells. It is believed the reduction of protofibrils may prevent the progression of AD by reducing damage to neurons in the brain and cognitive dysfunction.12
Investor Contacts:
| Eisai Co., Ltd. |
Eisai Inc. (U.S.) |
Eisai Europe, Ltd.
(UK, Europe, Australia, New Zealand and Russia) |
Public Relations Department
TEL: +81 (0)3-3817-5120 |
Libby Holman
+ 1-201-753-1945
Libby_Holman@Eisai.com |
EMEA Communications Department
+44 (0) 786 601 1272
EMEA-comms@eisai.net |
[Notes to editors]
1. About Lecanemab (generic name, U.S. brand name: LEQEMBI®),
Lecanemab is the result of a strategic research alliance between Eisai and BioArctic. Lecanemab is a humanized immunoglobulin gamma 1 (IgG1) monoclonal antibody directed against aggregated soluble (protofibril) and insoluble forms of amyloid-beta (Aβ). In the U.S., LEQEMBI was granted traditional approval by the U.S. Food and Drug Administration (FDA) on July 6, 2023. LEQEMBI is an amyloid beta-directed antibody indicated as a disease-modifying treatment for Alzheimer’s disease (AD) in the U.S. Treatment with LEQEMBI should be initiated in patients with mild cognitive impairment (MCI) or mild dementia stage of disease, the population in which treatment was initiated in clinical trials. There are no safety or effectiveness data on initiating treatment at earlier or later stages of the disease than were studied. In Japan, Eisai received approval from the Ministry of Health, Labour and Welfare (MHLW) on September 25, 2023 to manufacture and market lecaenmab as a treatment for slowing progression of MCI and mild dementia due to AD.
Please see full Prescribing Information, including Boxed WARNING in the United States.
Eisai has also submitted applications for approval of lecanemab in EU, China, Canada, Great Britain, Australia, Switzerland, South Korea and Israel. In China and Israel, the applications have been designated for priority review, and in Great Britain, lecanemab has been designated for the Innovative Licensing and Access Pathway (ILAP), which aims to reduce the time to market for innovative medicines.
Eisai has completed a lecanemab subcutaneous bioavailability study, and subcutaneous dosing is currently being evaluated in the Clarity AD (Study 301) open-label extension (OLE). A maintenance dosing regimen has been evaluated as part of Study 201.
Since July 2020 the Phase 3 clinical study (AHEAD 3-45) for individuals with preclinical AD, meaning they are clinically normal and have intermediate or elevated levels of amyloid in their brains, is ongoing. AHEAD 3-45 is conducted as a public-private partnership between the Alzheimer’s Clinical Trial Consortium that provides the infrastructure for academic clinical trials in AD and related dementias in the U.S, funded by the National Institute on Aging, part of the National Institutes of Health, Eisai and Biogen.
Since January 2022, the Tau NexGen clinical study for Dominantly Inherited AD (DIAD), that is conducted by Dominantly Inherited Alzheimer Network Trials Unit (DIAN-TU), led by Washington University School of Medicine in St. Louis, is ongoing and includes lecanemab as the backbone anti-amyloid therapy.
2. About E2511
E2511 is Eisai’ s in-house discovered and developed investigational novel molecule that directly binds to tropomyosin receptor kinase A (TrkA); a nerve growth factor (NGF) located on the neural cell membrane. E2511 could potentially promote recovery and synaptic remodeling of damaged cholinergic neurons. A Phase 1 study for E2511 is underway.
3. About the Collaboration between Eisai and Biogen for Alzheimer’s Disease
Eisai and Biogen have been collaborating on the joint development and commercialization of AD treatments since 2014. Eisai serves as the lead of lecanemab development and regulatory submissions globally with both Eisai and Biogen co-commercializing and co-promoting the product and Eisai having final decision-making authority. In Japan, Eisai and Biogen Japan will co-promote lecanemab, with Eisai distributing the product as the Marketing Authorization Holder.
4. About the Collaboration between Eisai and BioArctic for Alzheimer’s Disease
Since 2005, Eisai and BioArctic have had a long-term collaboration regarding the development and commercialization of AD treatments. Eisai obtained the global rights to study, develop, manufacture and market LEQEMBI for the treatment of AD pursuant to an agreement with BioArctic in December 2007. The development and commercialization agreement on the antibody LEQEMBI back-up was signed in May 2015.
References
1LEQEMBI US Prescribing Information under Traditional Approval
4van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21.
5Brendza RP, et al. Anti-Aβ antibody treatment promotes the rapid recovery of amyloid-associated neuritic dystrophy in PDAPP transgenic mice
J Clin Invest. 2005;
115(2):428-433.
https://doi.org/10.1172/JCI23269.
6Ono K, Tsuji M. Protofibrils of Amyloid-β are Important Targets of a Disease-Modifying Approach for Alzheimer’s Disease. Int J Mol Sci. 2020;21(3):952. Doi: 10.3390/ijms21030952. PMID: 32023927; PMCID: PMC7037706.
7Söderberg L, et al. Lecanemab, Aducanumab, and Gantenerumab — Binding Profiles to Different Forms of Amyloid‑Beta Might Explain Efficacy and Side Effects in Clinical Trials for Alzheimer’s Disease.
Neurotherapeutics 2023 20:195–206
https://doi.org/10.1007/s13311-022-01308-6 Accessed October 12, 2023.
8Hartley DM, Walsh DM, Ye CP, Diehl T, Vasquez S, Vassilev PM, Teplow DB, Selkoe DJ. Protofibrillar intermediates of amyloid beta-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J Neurosci. 1999;19(20):8876-84. doi: 10.1523/JNEUROSCI.19-20-08876.1999. PMID: 10516307; PMCID: PMC6782787.
10Chen, Gf., Xu, Th., Yan, Y. et al. Amyloid beta: structure, biology and structure-based therapeutic development.
Acta Pharmacol. 2017;38:1205.
https://doi.org/10.1038/aps.2017.28
11Habashi M., Vulta S., Tripathi K., et al. Early diagnosis and treatment of Alzheimer’s disease by targeting toxic soluble Aβ oligomers. Biophysics and Computational Biology. 2022;10.1073. https://www.pnas.org/doi/epdf/10.1073/pnas.2210766119
12Amin L, Harris DA. Aβ receptors specifically recognize molecular features displayed by fibril ends and neurotoxic oligomers. Nat Commun. 2021;12:3451. doi:10.1038/s41467-021-23507-z

by codm | Oct 11, 2023 | Newsletter
For Print (PDF)
Eisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito, “Eisai”) announced today the presentation of research across various types of cancer from its oncology portfolio and pipeline during the European Society for Medical Oncology (ESMO) Congress 2023, which is taking place virtually and in-person in Madrid, Spain from October 20 to 24.
Notable presentations include a post-hoc analysis of tumor response by baseline characteristics of the metastases from the pivotal Phase 3 CLEAR (Study 307)/KEYNOTE-581 trial, which evaluated lenvatinib (LENVIMA®), the orally available multiple receptor tyrosine kinase inhibitor discovered by Eisai, plus pembrolizumab (KEYTRUDA®), anti-PD-1 therapy from Merck & Co., Inc., Kenilworth, versus sunitinib for the first-line treatment of patients with advanced renal cell carcinoma (NCT02811861; Presentation: #1903P). An exploratory analysis from the pivotal Phase 3 Study 309/KEYNOTE-775 trial of outcomes for patients with advanced endometrial cancer who completed treatment with pembrolizumab and continued with lenvatinib will also be presented (NCT03517449; Presentation: #748P).
“As a research and development-focused company driven by our hhc (human health care) concept, we strive to make a difference in the lives of patients and their families by advancing the science of cancer medicine with our robust portfolio and pipeline,” said Dr. Takashi Owa, Chief Scientific Officer, Senior Vice President, Eisai Co., Ltd. “At this year’s ESMO meeting, analyses from the pivotal Phase 3 CLEAR and Study 309/KEYNOTE-775 trials may provide greater insights into the treatment of patients with advanced renal cell carcinoma and certain types of advanced endometrial carcinoma. We also look forward to sharing data for lenvatinib and from our pipeline, as well as engaging in critical scientific exchange with the community in service of moving oncology research forward.”
Additional data from the LEAP (LEnvatinib And Pembrolizumab) clinical program to be presented include safety-run-in results from the Phase 3 LEAP-014 trial evaluating lenvatinib plus pembrolizumab and chemotherapy as a treatment option for patients with metastatic esophageal squamous cell carcinoma (NCT04949256; Presentation: #1534P). A network meta-analysis of lenvatinib versus key comparators as first-line treatment for patients with unresectable hepatocellular carcinoma will also be presented during a poster session (Presentation: #1007P).
Research from Eisai’s pipeline will be featured in a poster presentation of findings from the dose-expansion portion of a Phase 1 study evaluating E7389-LF, a liposomal formulation of eribulin, as a potential first-line chemotherapy treatment option for patients with metastatic/advanced HER2-negative breast cancer (Presentation: #405P). Additionally, insights from preclinical research on farletuzumab ecteribulin (FZEC, formerly known as MORAb-202), a folate receptor alpha (FRα)-targeting antibody drug conjugate (ADC), in endometrial cancer will be presented (Presentation: #786P).
This release discusses investigational compounds and investigational uses for FDA-approved products. It is not intended to convey conclusions about efficacy and safety. There is no guarantee that any investigational compounds or investigational uses of FDA-approved products will successfully complete clinical development or gain FDA approval.
The full list of presentations is included below. These abstracts will be made available via the ESMO website on Monday, October 16, 2023, at 12:05 AM CEST.
| Cancer Type |
Study/
Compound |
Abstract Title |
Abstract Type & Details |
| Lenvatinib Plus Pembrolizumab |
| Genitourinary Cancer |
CLEAR |
Tumor response by baseline metastases in patients with renal cell carcinoma treated with lenvatinib plus pembrolizumab vs sunitinib: post hoc analysis of the CLEAR trial |
Poster Session
Presentation #1903P
October 23, 2023
9:00 AM-5:00 PM CEST
|
| Gynecologic Cancer |
Study 309/
KEYNOTE-775 |
Outcomes for patients with advanced endometrial cancer who completed pembrolizumab and continued lenvatinib in the phase 3 Study 309/KEYNOTE-775 |
Poster Session
Presentation #748P
October 22, 2023
9:00 AM-5:00 PM CEST
|
| Gastrointestinal Cancer |
LEAP-014 |
First-line lenvatinib plus pembrolizumab and chemotherapy for metastatic esophageal squamous cell carcinoma: safety run-in results from the phase 3 LEAP-014 study |
Poster Session
Presentation #1534P
October 23, 20239:00 AM-5:00 PM CEST
|
| Lenvatinib |
| Gastrointestinal Cancer |
Network Meta-Analysis |
Network meta-analysis of lenvatinib vs key comparators in first-line unresectable hepatocellular carcinoma
|
Poster Session
Presentation #1007P
October 23, 2023
9:00 AM-5:00 PM CEST
|
| Real-World Evidence_
Liver Cancer |
Safety and efficacy of lenvatinib in patients with unresectable hepatocellular carcinoma in real-world practice in Korea |
Poster Session
Presentation #987P
October 23, 2023
9:00 AM-5:00 PM CEST
|
| Eribulin |
| Breast Cancer |
Eribulin |
Health outcomes of treatment sequences with eribulin or other single agents’ chemotherapy for treating relapsed metastatic HER2-negative breast cance
|
Poster Session
Presentation #462P
October 21, 2023
9:00 AM-5:00 PM CEST
|
| Pipeline |
| Breast Cancer |
E7389-LF |
E7389-LF as a first-line chemotherapy for patients with metastatic/advanced HER2-negative breast cancer: Results from a phase 1 study dose-expansion part |
Poster Session
Presentation #405P
October 21, 2023
9:00 AM-5:00 PM CEST
|
| Gynecologic Cancer |
Farletuzumab Ecteribulin
(FZEC) |
Antitumor activity of farletuzumab ecteribulin in a panel of endometrial cancer patient-derived xenografts with four different molecular subtypes |
Poster Session
Presentation #786P
October 22, 2023
9:00 AM-5:00 PM CEST
|
In March 2018, Eisai and Merck & Co., Inc., Rahway, NJ, USA (known as MSD outside the United States and Canada), through an affiliate, entered into a strategic collaboration for the worldwide co-development and co-commercialization of lenvatinib, both as monotherapy and in combination with the pembrolizumab, anti-PD-1 therapy from Merck & Co., Inc., Kenilworth, NJ, USA. Eisai and Merck & Co., Inc., Kenilworth, NJ, USA are studying the lenvatinib plus pembrolizumab combination through the LEAP (LEnvatinib And Pembrolizumab) clinical program in various tumor types across multiple clinical trials.
In June 2021, Eisai and Bristol Myers Squibb entered into an exclusive global strategic collaboration agreement for the co-development and co-commercialization of FZEC. Eisai and Bristol Myers Squibb are currently investigating FZEC in multiple studies including: a Phase 1/2 clinical study for select solid tumors including endometrial cancer, a Phase 2 clinical study for non-small cell lung cancer, and a Phase 2 clinical study for ovarian cancer, peritoneal cancer and fallopian tube cancer.
Media Inquiries:
Public Relations Department,
Eisai Co., Ltd.
+81-(0)3-3817-5120
[Notes to editors]
1. Eisai’s Focus on Cancer
Eisai acknowledges “Oncology” as one of its key strategic areas, and will continue to focus on the discovery and development of anti-cancer drugs within drug discovery domains including “tumor microenvironment”, “proteostasis disruption”, “cell linage and cell differentiation”, and “inflammation, hypoxia, oxidative stress and cell senescence” under the Deep Human Biology Learning (DHBL) drug discovery and development organization. Eisai aspires to discover innovative new drugs with new targets and mechanisms of action from these domains with the aim of contributing to the cure of cancers.
* KEYTRUDA® is a registered trademark of Merck Sharp & Dohme LLC., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.

by codm | Oct 4, 2023 | Newsletter
For Print (PDF)
Company Name: Eisai Co., Ltd.
Representative: Haruo Naito,
Representative Corporate Officer and CEO
(Code No. 4523 Tokyo Stock Exchange Prime Market)
Inquiries: Sayoko Sasaki, Vice President
Corporate Communications
(Phone +81-3-3817-5120)
Eisai Co., Ltd. (Head Office: Tokyo; Representative Corporate Officer and CEO: Haruo Naito; the “Company” or “Eisai”) hereby announces that the Company has today decided to absorb and merge with KAN Research Institute, Inc., the Company’s wholly owned subsidiary (Address: Hyogo Prefecture; “KAN Research Institute”) on April 1, 2024 (the “Merger”). Since the Merger is a simplified absorption-type merger targeting the Company’s wholly owned subsidiary, certain disclosure items and details have been omitted.
1. Purpose of the Merger
In October 2022, the Company group’s research and development (R&D) organization transitioned to the Deep Human Biology Learning (DHBL) drug discovery and development. Under the DHBL drug discovery and development, Eisai views disease as a continuum and is redefining the disease concept through comprehensive analysis of the genomic, pathophysiological and clinical information associated with the root cause of the disease. Thereafter, Eisai will deepen its knowledge of human biology by acquiring data such as biomarker and imaging data, obtained from patients who have taken the Company’s drugs, which will lead to next-generation drug discovery, and thereby aim to create innovative next-generation drug discovery concepts.
KAN Research Institute was established in 1997, as a separate corporation that can independently operate in order to promote drug discovery activities by taking an approach that is different from that of the Company. KAN Research Institute has succeeded in its own technology development concerning the acquisition of antibodies, drug delivery to the brain, generation of genetically modified animal models, etc.
Meanwhile, under the DHBL drug discovery and development, the Company has been promoting the integrated management of facilities and costs, and effective deployment of human resources in Eisai group’s R&D related organizations and, in order to fully utilize the human resources, technology, facilities, etc., of KAN Research Institute, the Company decided to absorb and merge with KAN Research Institute. Through this merger, Eisai will further deepen it’s understanding of human biology, while KAN Research Institute will continue to contribute to the innovative creation as a major base of drug discovery research of the Company.
2. Summary of the Merger
(1) Schedule of the Merger
| Approval of the Merger Agreement by the Executive Committee |
October 4, 2023 |
| Signing of the Merger Agreement |
October 4, 2023 |
| Date of the Merger (Effective Date) |
April 1, 2024 |
(Note) The Merger constitutes a simplified merger pursuant to Article 796, Paragraph (2) of the Companies Act on the part of the Company and a short-form merger pursuant to Article 784, Paragraph (1) of the Companies Act on the part of KAN Research Institute. Therefore, the Merger is conducted without obtaining the approval of either company’s general shareholders meeting regarding the Merger Agreement.
(2) Method of the Merger
The Merger is an absorption-type merger with the Company as the surviving company and KAN Research Institute as the absorbed company which dissolves as a result of the Merger.
(3) Details of allocation related to the Merger
Since the Company holds all shares in KAN Research Institute, no consideration will be provided upon the Merger.
(4) Treatment of share options and bonds with share options associated with the Merger
Not applicable.
3. Overview of the companies involved in the Merger (as of March 31, 2023)
|
Company Survivingthe Absorption-Type Merger |
Company Absorbed in the Absorption-Type Merger |
| (1)Trade Name |
Eisai Co., Ltd. |
KAN Research Institute, Inc. |
| (2)Address of Head Office |
4-6-10 Koishikawa, Bunkyo-ku, Tokyo |
6-8-2 Minatojima-minamimachi, Chuo-ku, Kobe, Hyogo |
| (3)Representative |
Haruo Naito, Representative Corporate Officer and CEO |
Teiji Kimura, President & CEO |
| (4)Scope of Business |
Research and development, manufacture, sale and import and export of pharmaceuticals |
Discovery research of pharmaceuticals and research on life sciences |
| (5)Capital |
44,986 million yen |
70 million yen |
| (6)Date of Incorporation |
December 6, 1941 |
April 25, 1997 |
| (7)Number of Issued Shares |
296,566,949 Shares |
1,400 Shares |
| (8)End of Fiscal Year |
End of March |
End of March |
| (9)Major Shareholders and Shareholding Ratios (Note) |
The Master Trust Bank of Japan, Ltd. (Trust Account) 19.31% |
Eisai Co., Ltd. 100.00% |
| Custody Bank of Japan, Ltd. (Trust Account))12.61% |
| State Street Bank and Trust Company 505001 7.15% |
| Nippon Life Insurance Company 3.00% |
| Saitama Resona Bank, Limited 1.85% |
| (10)Financial Position and Business Performance for the Most Recent Fiscal Year |
| Fiscal year |
FY3/2023
(Consolidated; IFRS) |
FY3/2023
(Non-consolidated; J-GAAP)
|
| Equity attributable to owners of the parent / Net assets |
799,959 million yen |
707 million yen |
| Total assets |
1,263,350 million yen |
1,438 million yen |
| Equity per share attributable to owners of the parent / Net assets per share |
2,789.32 yen |
504,784.57 yen |
| Revenue / Net sales |
744,402 million yen |
1,816 million yen |
| Operating income |
40,040 million yen |
74 million yen |
| Income before income taxes / Net income before income taxes |
45,012 million yen |
76 million yen |
| Income attributable to owners of the parent / Net income |
55,432 million yen |
49 million yen |
| Basic earnings per share / Net income per share |
193.31 yen |
35,093.10 yen |
(Note) The shareholding ratios are calculated after deducting the treasury shares from the total number of issued shares.
4. Status after the Merger
As a result of the Merger, there will be no changes in the trade name, business scope, address of the head office, representative, capital or fiscal year of the Company.
5. Future outlook
The impact of the Merger on the Company’s consolidated financial results will be minor because the Merger is a merger with the Company’s wholly owned subsidiary.

by codm | Sep 28, 2023 | Newsletter
For Print (PDF)
Tokio Marine & Nichido Fire Insurance Co., Ltd.
Eisai Co., Ltd.
Tokio Marine & Nichido Fire Insurance Co., Ltd. (Headquarters: Tokyo, CEO: Shinichi Hirose, “Tokio Marine Nichido”) and Eisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito, “Eisai”) announced that they have co-developed “Dementia Care Support Insurance” to financially support early detection and early treatment for dementia as a part of their business alliance for the realization of a Dementia Inclusive Society.
|
1. Background
Tokio Marine Nichido and Eisai concluded a business alliance agreement for the realization of a Dementia Inclusive Society in August 2019, and the two companies have promoted initiatives to achieve this purpose, including the provision since April 2021 of “NouKNOW®※1”, a digital tool for self-assessment of cognitive function developed by Eisai, as an ancillary service for “Long-term care indemnity (installment payment)”, sold by Tokio Marine Nichido.
Dementia was thought to be difficult to treat, but with the approval of a new drug for Alzheimer’s disease, which accounts for 60% of dementia cases, and the hope of slowing disease progression by starting treatment in the early stages of disease, the preparation for early detection and early treatment is becoming increasingly important.
Amid such circumstances, the two companies co-developed a new insurance product, “Dementia Care Support Insurance” to financially support early detection and early treatment of the disease, with the expertise Tokio Marine Nichido has developed through dealing with insurance products and related services and Eisai’s extensive experience in the field of dementia.
※1 A tool for checking brain reaction speed, attention, visual learning and memory through four types of simple tests with images of playing cards on a PC, tablet, or smartphone. (Not a medical device)
2. Overview of (Industry’s First※2) “Dementia Care Support Insurance”
(1) Details of Coverage/Service
The new treatment is indicated for patients with mild cognitive impairment and mild cognitive impairment due to Alzheimer’s disease, but determination of eligibility requires medical tests, including a PET scan, to confirm amyloid-β pathology. Since these tests and treatments require a certain level of out-of-pocket expenses, the following coverage will be provided for financial support.
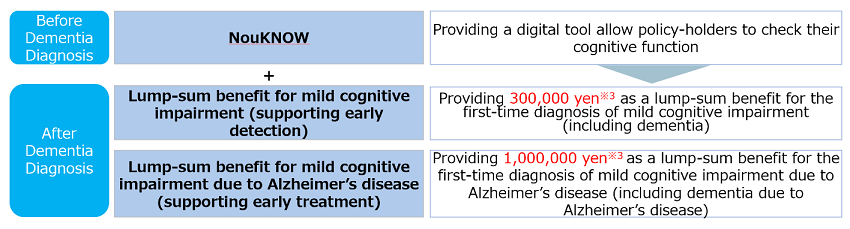
① Lump-sum benefit for mild cognitive impairment (Support for early detection)
In the event of the first diagnosis of mild cognitive impairment (including dementia), a lump sum benefit for the purpose of covering costs for amyloid PET tests, etc. is provided.
② Lump-sum benefit for mild cognitive impairment due to Alzheimer’s disease (Support for early stage treatment)
In the event of the first diagnosis of mild cognitive impairment due to Alzheimer’s disease (including dementia due to Alzheimer’s), a lump-sum benefit for the purpose of covering medical costs for treatment with the new drug is provided.
Additionally, the policy provides the opportunity to use “NouKNOW” as an ancillary service, ensuring the support of early detection and early treatment by identifying cognitive decline.

※2 The first coverage in the non-life insurance industry focusing on mild cognitive impairment due to Alzheimer’s disease and dementia due to Alzheimer’s disease (according to a survey conducted by Tokio Marine Nichido)
※3 May vary depending on terms of contract
(2) Process Upon Signing The Policy
Potential policy holders need to complete a “NouKNOW” test to purchase the policy, along with completing a Statement of Health form.
(3) Insurance Premium
Monthly 1,370 yen (in the case of a male aged 50 to 54 years receiving a 300,000 yen lump-sum benefit for mild cognitive impairment, and 1 million yen for mild cognitive impairment due to Alzheimer’s disease). *May vary depending on terms of contract
(4) Type of Policy
A group insurance in which a large-scale entity, such as a company, purchases the policy and its members voluntarily purchase the coverage through the entity.
3. Future Perspectives
Tokio Marine Nichido and Eisai will further promote efforts to resolve various social challenges by expanding our network through collaboration with various companies and organizations, to realize a Dementia Inclusive Society.
Media Contacts:

by codm | Sep 28, 2023 | Newsletter
For Print (PDF)
Eisai Co., Ltd. (Head office: Tokyo, CEO: Haruo Naito, “Eisai”) announced today that it has launched a new “Innovation” page on its corporate website. The “Innovation” page is comprised of four sections: “Research & Development (R&D),” “Ecosystem,” “Open Innovation,” and “Corporate Venture Capital,” which are the core of Eisai’s innovation creation. Each part introduces information such as Eisai’s strengths, originality, and the status of specific initiatives.
Eisai’s Corporate Concept is “to give first thought to patients and people in the daily living domain, and to increase the benefits that health care provides.” Under this Concept, (also known as our human health care (hhc) Concept), we aim to effectively achieve social good in the form of relieving anxiety over health and reducing health disparities.
On the newly established “Innovation” page, Eisai has enhanced information on policies, characteristics, and examples of initiatives for innovation creation aimed at realizing it’s corporate concept. The “R&D” section contains new contents, such as an overview of the Deep Human Biology Learning (DHBL) drug discovery and development system, which was established last year, track records of Eisai’s long-term commitment to dementia, oncology and tropical diseases, and the current status of digital technology application in R&D settings. In addition, the “Ecosystem” section explains Eisai’s policy of evolving into an hhceco (hhc concept + ecosystem) company to empower people to “realize their fullest lives” how they would like, from the time that they are in good health up to their final moments, and the dementia hhceco model on which Eisai is focusing. Moreover, the “Open Innovation” and “Corporate Venture Capital” sections introduce their respective business policies and achievements, and an inquiry page for new partnering opportunities has been established.
Eisai will continue to disclose information in a proactive and easy-to-understand manner through our corporate website and communicate with all of our stakeholders to help them understand our corporate concept and business activities.
Media Inquiries:
Public Relations Department,
Eisai Co., Ltd.
+81-(0)3-3817-5120